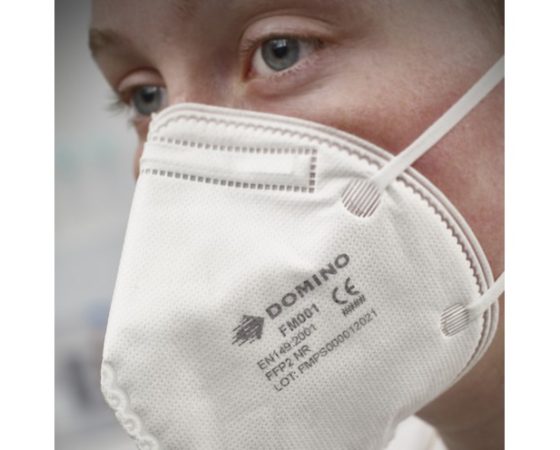
Domino’s new food packaging ink supports coding on respirator face masks
Fast-drying, high-contrast, and suitable for use on medical devices and personal protective equipment (PPE), TIJ-BK119 is the latest component in a range of specialist coding and marking solutions supporting the fight against COVID-19 from Domino Printing Sciences (Domino).
The current pandemic has seen many changes in the application of manufacturing technology and consumables – and BK119 is another example to add to the list. This newly launched ink for Domino’s Gx-Series range of high-quality, thermal inkjet (TIJ) printers was originally developed for food industry applications meaning it is food-packaging safe, and adheres to EuPIA’s Good Manufacturing Practice protocols. The food packaging ink is now being used by manufacturers of particle-filtering respirator face masks produced to protect frontline workers during the COVID-19 pandemic.
“Both respirator face masks and surgical masks have played a key role in the global fight against COVID-19,” says James Gibbins, Product Manager – Fluids, Domino. “In the last year, we have seen a significant rise in demand for these products, with many manufacturers producing them for the very first time.”
“Respirator face masks are classified as personal protective equipment, while surgical masks fall under the category of medical devices – both are subject to compulsory coding requirements within the countries in which they are sold,” continues Gibbins. “The capability and expertise to apply the necessary safety and quality codes are crucial, both for legislative compliance and consumer safety.”
With the global surgical mask market forecast to reach $87.67 billion by 2027[1], driven by the profound change in public health awareness, demand for coding and marking in the medical device sector is expected to remain high. With years of experience providing solutions to the medical device industry, Domino will continue to invest in its products, consumables, and services as the coding requirements for PPE evolve.
Unlike surgical masks, which are designed to restrict the spread of saliva droplets, and so protect others and the external environment from possible contamination, respirator face masks are designed to protect the wearer, and others, by filtering harmful substances and viruses from air flow in both directions.
Typically, TIJ is the coding application of choice for both respirator and surgical masks, as legibility and contrast are of key concern when adhering to regulatory standards, such as the European Union Regulation 2016/425 on Personal Protective Equipment, and Regulation 2017/745 on Medical Devices. Respirator face masks are subject to ISO and EN standards, which specify that each mask must carry a ‘CE logo’ and include reference to the filter type (e.g., FFP1, FFP2 and FFP3 in Europe) – which differs based on the region of sale. Among TIJ inks, BK119 is exceptionally high contrast for maximum legibility, making it suitable for all regulatory coding types, including Data Matrix and 2D codes.
“BK119 was developed to ensure maximum code quality, and legibility,” says Gibbins. “In addition to meeting compliance requirements of manufacturers of medical devices and PPE, it is also fast drying with excellent adhesion properties, making it an ideal coding solution for non-porous respirator face masks.”
Respirator masks are typically produced on high-speed manufacturing lines, with coding applied before masks are die-cut into their final form. This process can mean that masks interact with a lot of machinery after the code has been applied, both during die-cutting, and subsequent packaging. Such processes require a robust, fast-drying ink, to reduce the risk of a code becoming smudged or unreadable.
The face masks are made up of four, non-porous, layers, including a polypropylene filter and acrylic backing plate, and, depending on the filtration level, may also include moisture barriers for additional protection against airborne contagions. Respirator face masks cannot readily absorb water-based ink, and so ethanol-based BK119 is a highly suitable solution. For other, porous mask types, including surgical masks, Domino’s proprietary, water-based, food packaging inks (BK652 and BK651) provide an appropriate alternative.
“BK119 highlights Domino’s ongoing commitment to helping manufacturers through COVID-19 and beyond,” says Volker Watzke, EU Medical Devices Sector Manager, Domino. “Throughout the pandemic manufacturers have been faced with the need to adapt to the current circumstances, and many have stepped up to produce medical equipment for the first time.”
“We are pleased to be able to support the supply of significant volumes of face masks, COVID-19 test kits and other PPE globally by rapidly developing and adapting both products and consumables to meet the demands of our customers,” continues Watzke. “Our sector-related project management team works to meet the specific needs of individual customers in the medical and PPE sectors, with rapid sampling and implementation processes to meet evolving needs while adhering to industry standards”
To date, Domino has supplied over 1,000 TIJ printheads globally for face mask applications, with over 400 printheads going into a single factory in China. However, TIJ is not the only technology helping the fight against COVID-19. Domino has also supplied hundreds of V-Series thermal transfer overprinters, and Ax-Series continuous inkjet printers for coding onto the external packaging of surgical face masks. In addition to this, Domino has also seen a significant increase in demand for V-Series printers, and D-Series high-speed laser coders, for use in COVID-19 diagnostic test kit packaging applications. Since 1st January, more than 40 D-Series laser coders have been supplied for these applications in Korea alone.
Another rapidly growing application area for Domino is in the production and distribution of vaccines. As vaccine manufacturers struggle to increase capacity Domino is taking steps to deliver equipment around the world as fast as possible. One of China’s largest vaccine producers recently placed an urgent order for Domino M-Series labellers for coding vaccine packaging. Domino’s manufacturing and service organisations in Sweden and China worked to fulfil the order and have the machines, which represented several months of demand, air freighted and installed in under three weeks. Accuracy is crucial when it comes to vaccines, and so Domino is also working with customers to integrate vision solutions to verify codes and check the integrity of individual vials and syringes. As a global player with a leading position in the life sciences industry and a complete coding and marking solution portfolio Domino is committed to helping its customers around the world in this global fight.
“The last 12 months have brought about profound change on a global level, with manufacturers from across industries coming together to address public health needs,” says Jill Woods, Product Compliance Officer, Domino. “As a member of EuPIA, Domino’s manufactured inks – compliant to GMP – have enabled manufacturers to comply with the legal coding requirements for particle-filtering respirator face masks, as well as surgical masks, and additional PPE.”
“We are committed to helping our customers to navigate the challenges posed by the global pandemic. We will continue to demonstrate our expertise in formulating reliable, safe solutions in our advanced fluids manufacturing facilities in the UK and China, and work to ensure a steady supply of goods and services via our vast worldwide distribution network,” concludes Woods.
To discover more information on Domino’s solutions for medical devices, PPE, and medical-grade face masks, please visit https://bit.ly/2ZoL3N5.
[1] PR Newswire, ‘Recent Uptick in COVID-19 Outbreak is Driving Growth of Surgical Masks Demand’, https://www.prnewswire.co.uk/news-releases/recent-uptick-in-covid-19-outbreak-is-driving-growth-of-surgical-mask-demand-872302624.html
forrás: domino-printing.com 2021.02.22